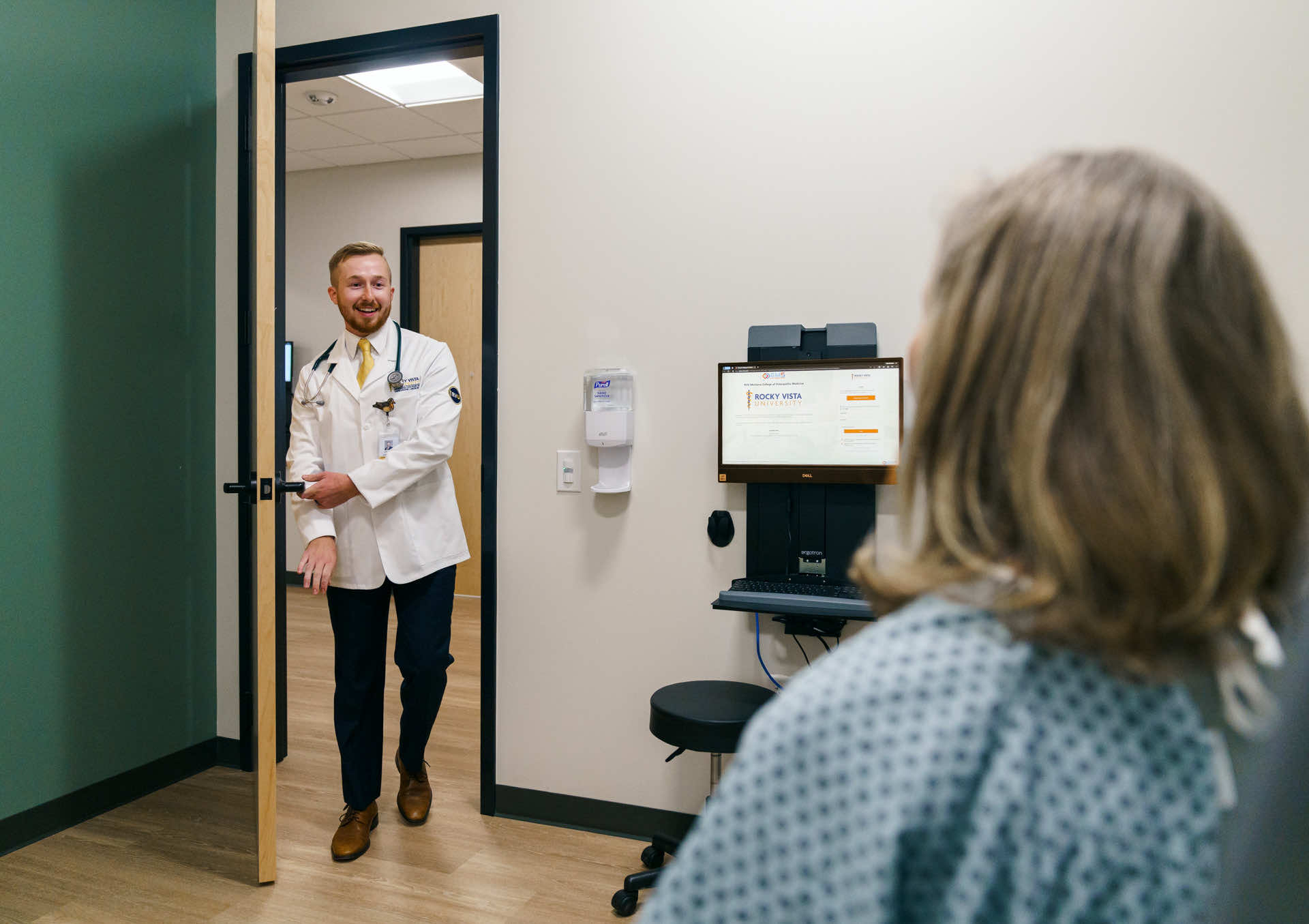
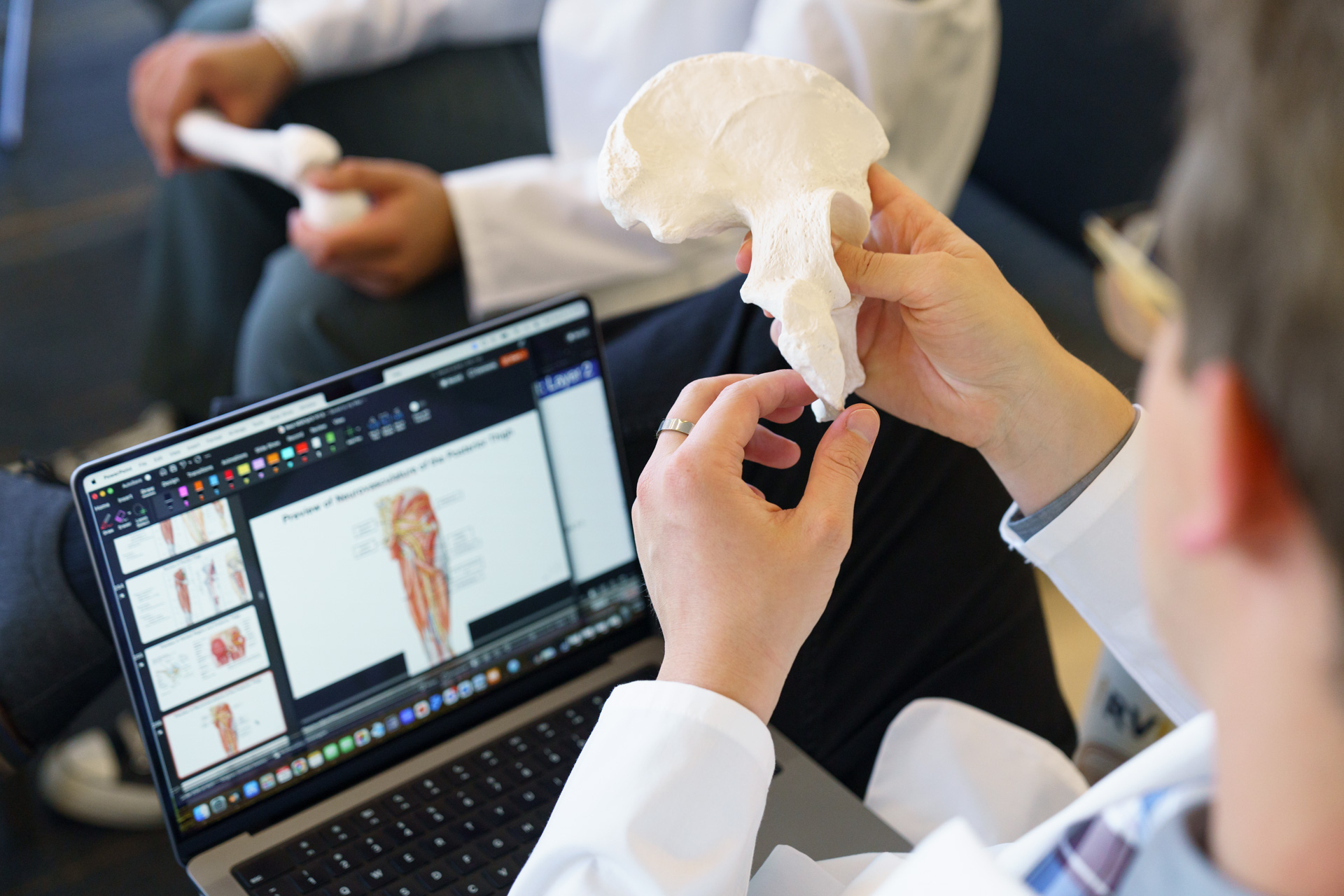
Students must be in good academic standing and are encouraged not to start research until after their first semester of medical school. Most students start by identifying a mentor and topic and then reviewing the literature as part of developing a detailed plan.
Office of Research and Scholarly Activity
A message from the Vice Provost of Research
Welcome to the Office of Research, where we are actively engaged in serving RVU’s growing research culture, which is fueled by curiosity, innovation, and our desire to advance medical education and evidence-based practices!
The mission of the Office of Research is to advance, promote, and celebrate all research and scholarly activities occurring within the Rocky Vista University community. We are committed to supporting the diversity and inclusion of all participants and of varied perspectives as an essential element to achieve this mission. Support is provided for all research areas, including basic biological, translational, educational, epidemiological, health equity, clinical, and advanced practice studies.
The Office of Research is committed to providing equitable access to guidance, resources, and opportunities for all faculty, staff, and students to engage in various research and scholarly activities that promote the advancement of educational, biomedical, and clinical sciences.
Equitable support for these areas includes expert mentorship, statistical consulting, an intramural grant program, travel funds, and other special events to facilitate networking.
Student research and scholarly activity is a vital part of health provider education. It supports and solidifies osteopathic principles and tenets in its interconnectedness to the practice of medicine. I invite you to explore our website and reach out to me with any questions or suggestions.
Happy Researching!
Amanda Brooks, PHD
Vice Provost of Research and Scholarly Activity
New Breakthroughs Await
Student research and scholarly activity is a vital part of the hands-on, real-world medical education experience that RVU emphasizes in our culture and curriculums.
The office of research is committed to providing equitable access to research opportunities that promote the advancement of educational, biomedical, and clinical sciences.

We support all areas of research including:
- basic biological
- translational
- educational
- epidemiological
- DEI-related
- clinical
- osteopathic practice studies
Equitable support for these areas includes:
- expert mentorship
- statistical consulting
- an intramural grant program
- travel funds
- special events to facilitate networking

Upcoming Events
MSBS Incoming Student Meetings
Join us for an exclusive virtual gathering designed for accepted students of the MSBS program! This is a great opportunity to connect with faculty, staff,…
Virtual
Research Weeks
Explore the results and explorations from Research Weeks over the years, when faculty, staff, and students come together to share ideas and insights.
Latest research news from the Office of Research and Scholarly Activity
RVU Research Strategic Plan 2023-2028
Dive into the key areas of focus that uphold the principles of our strong and growing research program.

Meet The Research Team

Amanda Brooks, PhD
Vice Provost of Research and Scholarly Activity, Director of Physician-Scientist Track (Utah)

Emily Cox
Senior Research Coordinator

Mita Das, PhD
Assistant Director of Research; Professor of Biochemistry

Jing Gao, MD, FAIUM
Ultrasound Lead/Director, Professor of Ultrasound

Shalese Gentry, Gentry
Administrative Assistant for Contract Research

Megan Lefevor
Administrative Assistant for Research

Isain Zapata, PhD
Assistant Director (Colorado Campus), Assistant Professor of Research and Statistics, Director of Introduction to Research Elective
Research FAQs
When do students generally start research?
How do I start research?
All research forms and process have been moved to the Research Hub. Use your SSO to login.
What does Research look like in Medical School?
- Literature reviews
- Secondary data analysis
- Survey studies / primary data collection
- Qualitative Research
- Qualitative data analysis
- Community based participatory research (or community outreach)
- Case reports/retrospective chart reviews
- Participant recruitment
- Abstract preparation for conferences or meetings
- Medical education research
- DEI research
What resources does RVU have available to do research?
- Internal grants
- Library resources including databases and literature review guidance
- Research specific software programs
- In house IRB system for human subjects’ research
- Healthcare clinics on CO and UT campuses that participate in clinical trials
- Basic wet lab areas available on each campus (equipped for sample storage/processing, simple assays)
- Travel fund to support presentation of your work at conferences
- Most publication open access charges covered
- Biostatistical and epidemiological support
- Seminars to teach students about research fundamentals
- Research week and other events throughout the year to showcase student research
- Engaged faculty and staff mentors
Can students work with entities outside of RVU?
Yes, absolutely. We have a pretty broad network or you can find / continue external projects. The RVU Research Office must be consulted for any project that includes external collaborations to ensure the highest level of integrity and safety.
Featured eBooks
Have questions? Want to meet?
Reach out and our team will be happy to answer your inquiries or set up a time to meet.
Latest Articles from our community
- General
Applying to RVU’s Master of Medical Science Program: A Step Toward Your Future in Healthcare
Read more- General
Protecting Pets, People, and Public Health: Rabies Prevention and the Role of Veterinary Education
Read more- General
Student Perspective: The Health Professions Scholarship Program
Read more
Awards